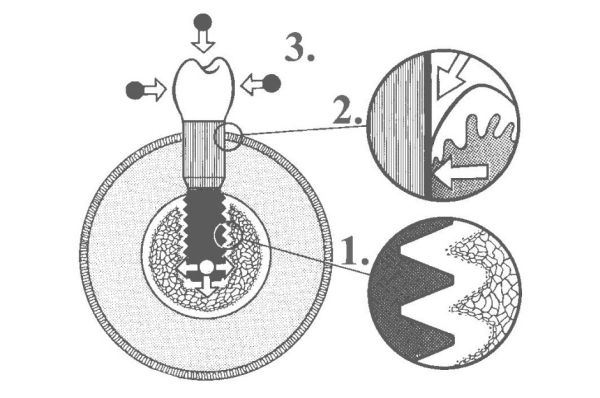
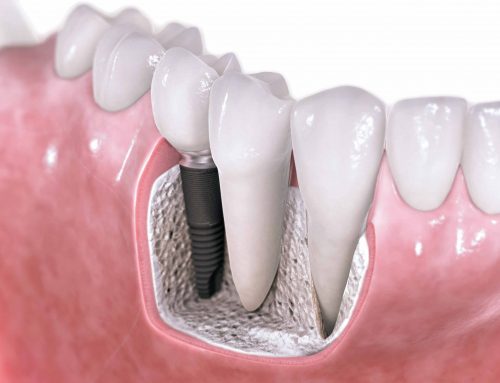
The biological fixation between the dental implant surfaces and jaw bones should be considered a prerequisite for the long-term success of implant-supported prostheses. There have been numerous studies indicating that implant surface roughness affects the rate of osseointegration (see Cell-to-Cell communication).
In this context, the implant surface engineering gained an important and decisive place in implant research over the last years. As the most investigated topic in, it aided the development of enhanced dental treatment modalities and the expansion of dental implant use. Dental implant manufacturers have modified implant surfaces through mechanical, chemical, electrochemical and laser treatments in their efforts to create implants that promote accelerated healing and osseointegration.
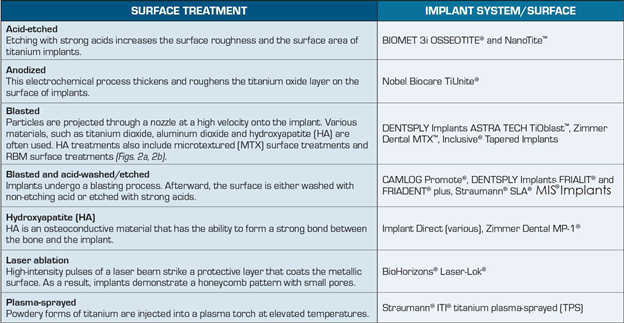
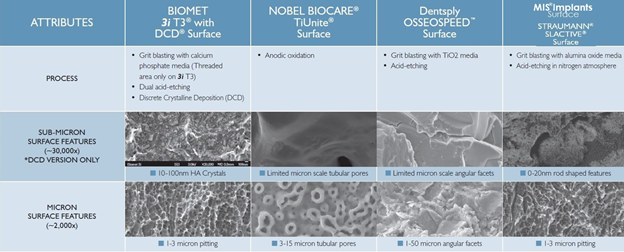
The main methods that are reported in the literature to create implant roughness are acid etching, sandblasting, titanium plasma spraying and hydroxyapatite (HA) coating. A current tendency is the manufacturing of implants with micro and submicron (nano) topography. Furthermore, the biofunctionalization of implants surfaces, by adding different substances to improve its biological characteristics, has also been recently investigated
This strategy aims at promoting the mechanism of osseointegration with faster and stronger bone formation, to confer better stability during the healing process, thus allowing more rapid loading of the implant.
Implant morphology influences bone metabolism: rougher surfaces stimulates differentiation, growth and attachment of bone cells, and increases mineralization.
Considering that different methods for implant surface engineering may lead to different and unique surface properties that might affect the host-to-implant response, it seems to be reasonable to test new implant surfaces as new biomaterials
Several review articles also suggest that regardless of surface treatments, most implants on the market are comparable to each other. There is no clear indication that dental implants with a specific morphological characteristic provide any benefits over implants with a different structure. Very minor differences exist in the performance of various implant surface types. Ongoing efforts in the development of surface technology are aimed at enhancing tissue-surface interactions and healing response, with the ultimate goal of improving patient care.
Finally, based on the state of the art of implant development, it is possible to predict that, within some time, implant surfaces coated with substances with biomimetic capacity will be available for clinical use. This process of biofuncionalization of implant aims at modulating new bone formation around implants, and it is the next step in implant development.
READ MORE:
http://www.ncbi.nlm.nih.gov/pubmed/23059581
http://www.jaypeejournals.com/eJournals/ShowText.aspx?ID=4329&Type=FREE&TYP=TOP&IN=~/eJournals/images/JPLOGO.gif&IID=337&isPDF=YES
http://www.mis-implants.com/upload/PDF/MNEWS_V3_(MC-MMAG5)_Rev3.pdf
http://scielo.br/pdf/bdj/v21n6/v21n6a01.pdf
http://www.glidewelldental.com/inclusive-magazine/volume5-2/implant-surface-treatment.aspx